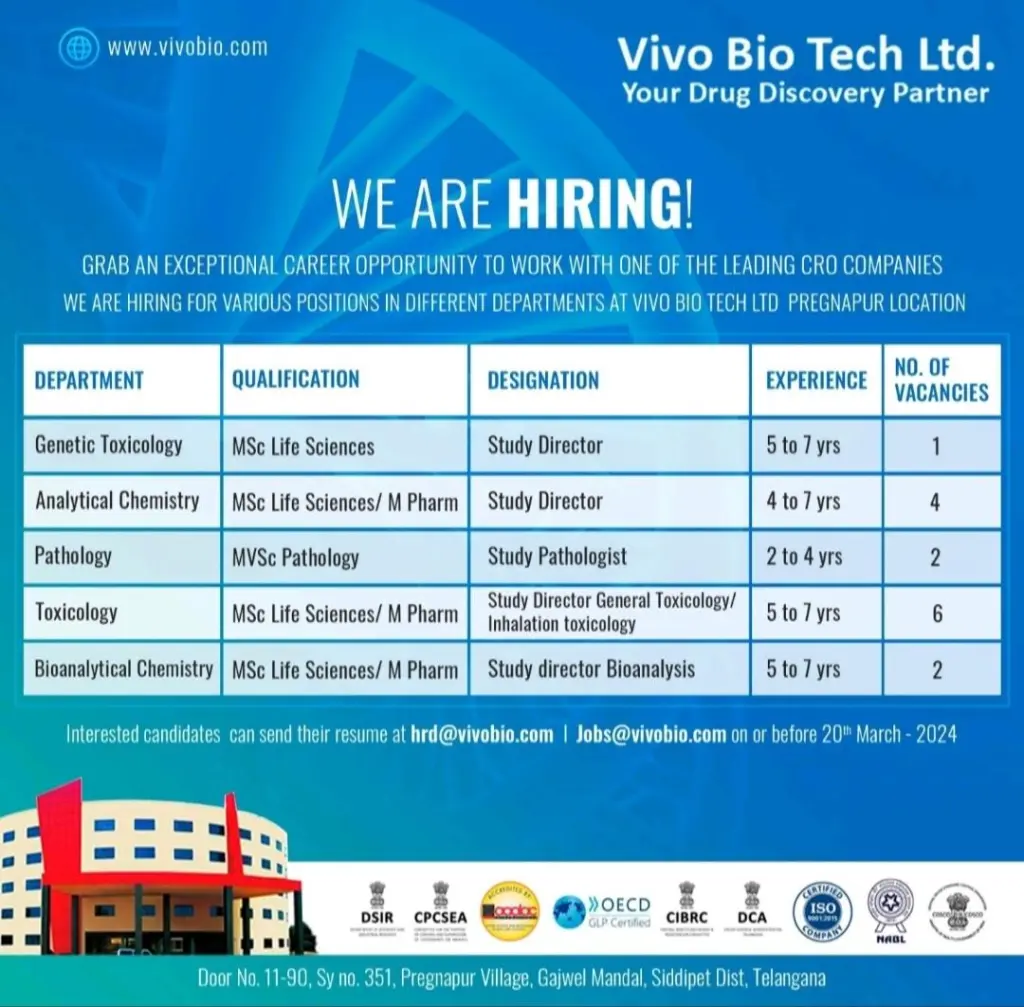
Vivo Bio Tech Ltd WALK IN DRIVE 2024 : Hiring As Genetic Toxicology & other role . MSc Life Sciences/ M Pharm Candidates can apply for the post.
Table of Contents
Vivo Bio Tech Ltd WALK IN DRIVE 2024 – Overview
- Company Website : www.vivobio.com
- Company – Vivo Bio Tech Ltd walkin
- Role – Genetic Toxicology & other role
- Qualification – MSc Life Sciences/ M Pharm
- Experience – 2-7 years
- Location – Telangana
- Salary- 3 LPA – 5 LPA
ABOUT Vivo Bio Tech Ltd COMPANY
Biotechnology companies like “Vivo Bio Tech” typically engage in activities such as drug discovery, development of biopharmaceuticals, genetic engineering, agricultural biotechnology, or industrial biotechnology. They may focus on areas such as healthcare, agriculture, environmental conservation, or industrial applications.
Without further context or specific details, it’s challenging to provide more information about “Vivo Bio Tech” or its activities. If you have specific questions or details about the company, such as its location, products, or services, please provide them, and I’ll do my best to assist you further. Alternatively, you may consider reaching out to the company directly or searching for information through industry databases or business directories.

Vivo Bio Tech Ltd WALK IN DRIVE 2024 – Genetic Toxicology Responsibilities
- Conducting Experiments: Planning, designing, and performing experiments to evaluate the genotoxicity of chemicals using various in vitro and in vivo assays.
- Data Interpretation: Analyzing experimental data and interpreting results to assess the genotoxic potential of chemicals, including determining the types and mechanisms of DNA damage induced.
- Sample Preparation and Analysis: Preparing biological samples (e.g., cells, tissues, or organisms) for genotoxicity testing and analyzing them using techniques such as the comet assay, micronucleus assay, Ames test, or other molecular biology methods.
- Quality Assurance and Compliance: Ensuring adherence to quality control measures, standard operating procedures (SOPs), and regulatory requirements (e.g., OECD guidelines) in all aspects of experimental work.
- Report Writing: Documenting experimental procedures, results, and conclusions in scientific reports or publications following established guidelines and standards.
- Collaboration and Communication: Collaborating with interdisciplinary teams, including toxicologists, pharmacologists, and regulatory affairs specialists, to integrate genetic toxicology data into overall safety assessments of chemicals. Effective communication of findings to stakeholders within and outside the organization is also essential.
- Literature Review: Staying updated on current research findings, scientific literature, and regulatory guidelines related to genetic toxicology to inform experimental design and interpretation.
- Method Development and Validation: Developing and validating new genetic toxicology assays or modifying existing protocols to improve sensitivity, specificity, and relevance for hazard identification and risk assessment.
| ALSO APPLY FOR Vodafone – Executive – view & apply Wells Fargo – Associate Operations Processor – view & apply Bny Mellon – Associate, Client Service – view & apply Qwidpro (WFH) – Virtual Assistant – view & apply |
Vivo Bio Tech Ltd WALK IN DRIVE 2024 – Skills Required
- Cell Culture Skills: Experience in maintaining cell lines and primary cells for in vitro genotoxicity assays, including knowledge of aseptic techniques and cell culture media preparation.
- Molecular Biology Techniques: Proficiency in various molecular biology techniques used for genotoxicity testing, such as DNA extraction, PCR, gel electrophoresis, and sequencing.
- Experimental Design: Ability to design and execute experiments effectively, including selecting appropriate assays, controls, and endpoints based on the research objectives and regulatory requirements.
- Knowledge of Toxicology: Understanding of basic toxicology principles, including dose-response relationships, mechanisms of toxicity, and the different types of DNA damage induced by chemical agents.
- Data Analysis: Proficiency in statistical analysis methods and software for analyzing genotoxicity data, interpreting results, and drawing valid conclusions.
Increase your selection chances Apply jobs base on your Location Qualification & Experience CLICK HERE
HOW TO APPLY FOR Vivo Bio Tech Ltd WALK IN DRIVE 2024 ?
To apply for the Vivo Bio Tech Ltd WALK IN DRIVE 2024– interested candidates must follow the procedure outlined below:
- Click on the “Apply here” button provided below. You will be redirected to Vivo Bio Tech Ltd WALK IN DRIVE 2024 company official career page.
- Click on “Apply Online”.
- If you have not registered before, create an account.
- After registration, login and fill in the application form with all the necessary details.
- Submit all relevant documents, if requested (e.g. resume, mark sheet, ID proof).
- Provide accurate information in your application.
- Verify that all the details entered are correct.
- Submit the application process after verification.
FOR Telangana LOCATION INTERESTED CANDIDATES CAN WALK-IN DIRECTLY TO THE BELOW VENUE FOR INTERVIEW
| Role : | Genetic Toxicology, Analytical Chemistry, Pathology, Toxicology, Bioanalytical Chemistry |
| Experience | 2 to 7 yrs |
| E.MAIL | Interested candidates can send their resume at [email protected] | [email protected] on or before 20th March -2024 |
| Address : | Door No. 11-90, Sy no. 351, Pregnapur Village, Gajwel Mandal, Siddipet Dist, Telangana |
Vivo Bio Tech Ltd WALK IN DRIVE 2024 – Frequently Asked Question ?
What is the Vivo Bio Tech Ltd selection process?
The selection process will be based on a Written test followed by Technical and HR interviews.
What is the average salary for the post?
The average salary is 3 LPA – 5 LPA for the this role.
What does vivo biotech do?
Vivo Bio Tech is a Hyderabad based public listed CRO offering services to biomedical industry worldwide in the areas of in-vitro / in-vivo toxicology, pharmacological investigations, analytical and physico-chemical testing.
Who is the founder of vivo bio tech ltd?
Dr.Sankaranarayanan is a discovery biologist with more than 35 years of experience in Pharmaceutical R&D. He has a proven track record in establishing drug discovery and development facilities, and implementation of GxP standards/accreditation for various biotech/pharma facilities.
What is vivo famous for?
Vivo Communication Technology Co. Ltd. is a Chinese multinational technology company headquartered in Dongguan, Guangdong that designs and develops smartphones, smartphone accessories, software and online services.
DISCLAIMER:
The Recruitment Information Provided above is for Informational Purposes only . The above Recruitment Information has been taken from the official site of the Organization. We do not provide any Recruitment guarantee. Recruitment is to be done as per the official recruitment process of the company. We don’t charge any fee for providing this job Information.












